

Cyclogest® (micronised progesterone)
What is Cyclogest®?
The preferred choice for Luteal Phase Support in 85% of IVF clinics surveyed in the UK.3
Supported across clinical trials with over 850 patients to have a demonstrated efficacy and safety profile.4,5
Demonstrated to have similar efficacy via both rectal and vaginal routes.6,7
Bioidentical to the progesterone produced by the patient’s body2 and micronised for optimised absorption.8,9
Cyclogest® 400mg UK Indications2:
- Luteal phase support (LPS) as part of assisted reproductive technology (ART) treatment for women.
- Premenstrual syndrome, including premenstrual tension and depression.
- Puerperal depression (post natal depression).
Cyclogest®can be an effective choice for Luteal Phase Support as part of ART treatment.10
The majority of patients undergoing ART cycles are affected by luteal phase defect, where the natural release of luteinising hormone (LH) and progesterone is inhibited following controlled ovarian hyperstimulation and oocyte aspiration. This negatively affects embryo implantation and development in the uterine lining and reduces the likelihood of a successful pregnancy.11
Administering exogenous progesterone, such as Cyclogest®, induces the secretory transformation of the endometrium to prepare the lining for embryo implantation through thickening of the endometrial lining and increasing vascularisation.12
Cyclogest® 200mg UK Indications2:
- Premenstrual syndrome, including premenstrual tension and depression.
- Puerperal depression (post natal depression).
Mechanism of action during Luteal Phase Support:
Cyclogest® is administered by vaginal insertion2 or rectal.
Cyclogest® is absorbed through the walls of the vagina.13
The active pharmaceutical ingredient, progesterone, is a steroid hormone and acts on multiple receptors, mostly binding to progesterone receptors (PGRs), to exert a pharmacological effect.14
Upon binding to the nuclear receptor, progesterone dimerises and is translocated to the nucleus to bind to a strand of DNA.14
The DNA binding allows for regulation of gene expression, which in turn helps embryo implanation and supports a pregnancy.14,15
Hormonal actions:
- Induces secretory transformation of the endometrium.
- Stabilises the endometrium.
- Stimulates the growth of the uterus.
- Maintains uterine quiescence by stabilising lysosomal membranes.
- Inhibits myometrial contractions.
Non-hormonal actions:
- Inhibits tissue rejection by modulation of the immune system.
- Protects the conceptus.
The clinical evidence for Cyclogest®
Cyclogest® supports successful embryo implantation
A 2018 randomised, observer-blind study involving 125 healthy individuals investigated the effects of various doses of Cyclogest® progesterone vaginal pessaries. Part 1 of the study compared Cyclogest® (200mg and 400mg) twice daily with progesterone vaginal gel (90mg) once daily. In Part 2, Cyclogest® (100mg) twice daily was compared with Cyclogest® (400mg) once daily. Placebo vaginal pessaries were also compared. Secretory transformation of the endometrium is
a surrogate marker for endometrial receptivity, thereby indicating an increased chance of implantation.5
The results demonstrated that Cyclogest® 400mg achieved the required endometrial response in 90-94% of women. Additionally, similar transformation of the endometrium to early and late secretory states across Cyclogest® 200mg administered twice daily, Cyclogest® 400mg administered twice daily and 90mg vaginal gel administered once daily was indicated.5 This is relevant given the growing evidence indicating a direct correlation between serum progesterone levels and pregnancy in frozen embryo transfer (FET) cycles with artificial endometrial preparation.17,18
A: Progesterone plasma concentrations after first dose application of different vaginal progesterone preparations on cycle Day 15 (A).
No Data Found
No Data Found
B: After repeated dose application on cycle days 24/25 (B).
No Data Found
No Data Found
bid, twice daily; od, once daily.
Adapted from: Duijkers et al., Hum Reprod 2018.5
Cyclogest® 400mg BID demonstrated an adequate endometrial response in 94% of women in a trial.
Cyclogest® supports the achievement of pregnancy
In a pivotal randomised phase III clinical trial involving 769 patients undergoing IVF, Cyclogest® 400mg achieved a clinical pregnancy rate (CPR) of 38.3% (FAS dataset) and 38.1% (PP dataset) after 38 days administration, indicating that Cyclogest® is an effective treatment for LPS in ART. Biochemical pregnancy and clinical implantation rates were comparable for both Cyclogest® 400mg vaginal pessaries and 90mg vaginal gel.10
No Data Found
No Data Found
Pregnancy rates (biochemical and clinical), Days 18, 38 and 70 in the Full Analysis Set (N = 737).
No Data Found
No Data Found
BPR, biochemical pregnancy rate; CPR, clinical pregnancy rate; FAS, full analysis set; PP, per protocol set. Three patients were included in the FAS but did not have Day 38 pregnancy information.
Adapted from: Saunders et al., Hum Reprod 2020.10
It has also been demonstrated that 400mg of micronised vaginal progesterone administered twice daily may increase the number of live births in women with early pregnancy bleeding and a previous miscarriage.19 These findings have led to recommendations by NICE to offer vaginal micronised progesterone 400mg twice daily to women with an intrauterine pregnancy confirmed by a scan, if they have vaginal bleeding and have previously had a miscarriage.20
Furthermore, data has shown that vaginal progesterone can help to reduce the risk of preterm birth before 34 weeks in women with a previous history of preterm birth, and in women with a short cervix (25mm or less). In these cases, progesterone treatment is recommended to start between 16+0 and 24+0 weeks of pregnancy and continue until at least 34 weeks. NICE therefore recommends that progesterone treatment should be offered as an equal option with cervical cerclage.21
Additional benefits of Cyclogest®
Cyclogest® contains a natural progesterone
Progesterone
Progesterone
molecule
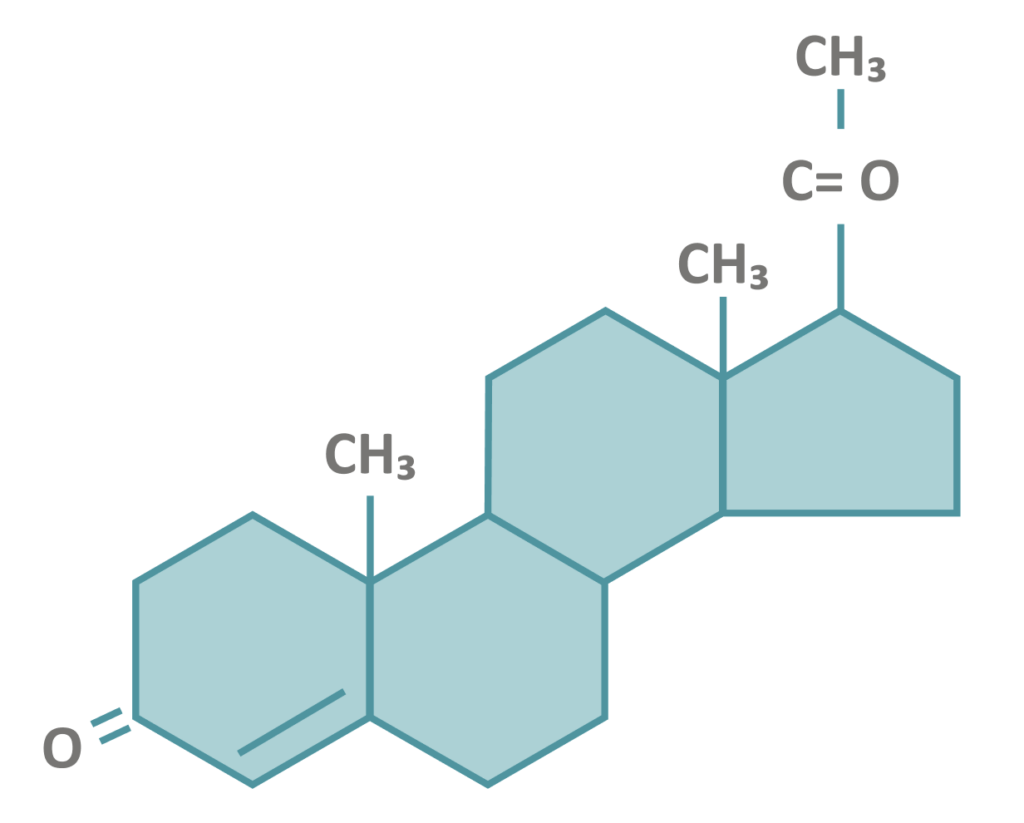
Progesterone in Cyclogest®
Bio-identical progesterone
molecule in Cyclogest®22

Adapted from: National Center for Biotechnology Information. PubChem Compound Summary for CID 5994, Progesterone 2022.24
Cyclogest® has shown to be well-tolerated
| Progesterone vaginal pessaries n=385 | Progesterone vaginal gel n=383 | Total n=768 | |
|---|---|---|---|
| Number of patients with any AE | 168 (43.6%) | 171 (44.6%) | 339 (44.1%) |
| Number of patients with any SAE | 6 (1.6%) | 13 (3.4%) | 19 (2.5%) |
AE, adverse event; SAE, serious adverse event.
Adapted from: Saunders et al., Hum Reprod 2020.10
Cyclogest® Benefits
- Cyclogest® is a self-lubricating pessary (Suppocire® BS2 creates a smooth outer layer) that is small and discreet.
- Cyclogest® can be inserted easily by the patient without the need of an applicator.
- Cyclogest® requires only twice daily administration2 to easily fit around a woman’s life.
- Cyclogest® can also be administered rectally, where vaginal administration is not possible.2
- Cyclogest® is suitable for vegan and vegetarians. This is certified with the Vegan Society.
Cyclogest® provides a higher dosage treatment option
Cyclogest® was shown to be the clinician's preferred treatment option in the UK
Reported first-choice preparation for luteal support.
No Data Found
Adapted from: Russell R et al., Hum Fertil 2015.3
Product information
- Cyclogest® 200 mg pessaries https://www.medicines.org.uk/emc/product/5568/smpc
- Cyclogest® 400 mg pessaries https://www.medicines.org.uk/emc/product/5569/smpc
Medicinal forms & pricing information for progesterone
HCP experiences with Cyclogest®
- Ahmed DT, Ahmed FT. Vaginal Progesterone For Maintenance Of Tocolysis In A Sample Of Iraqi Women. European Journal of Molecular & Clinical Medicine 2020;7:172-184. Available at: https://ejmcm.com/pdf_3765_4d4c4e66ee51daddc47877ad8c48bbf5.html [Last accessed: April 2022].
- Cyclogest 200mg and 400mg Summary of Product Characteristics. EMC. 2021. Available at: https://www.medicines.org.uk/emc/product/5568/smpc https://www.medicines.org.uk/emc/product/5569/smpc [Last accessed: Oct 2023].
- Russell R, Kingsland C, Alfirevic Z, Gazvani R. Duration of luteal support after IVF is important, so why is there no consistency in practice? The results of a dynamic survey of practice in the United Kingdom. Human Fertility 2015;18:43-47. Available at: https://www.tandfonline.com/doi/full/10.3109/14647273.2014.921337 [Last accessed: March 2022].
- Urbancsek J, Stamenov G, Kurecova B, Ljubic G, Magnusdottir TB, Hrafnsdottir S, Wargenau M, Klingmann I, D’Hooghe T. Randomized clinical trial to compare the pregnancy rates of vaginally applied progesterone 400 mg pessary and progesterone 8% gel after in-vitro fertilization. Human Reproduction 2017;32(Suppl 1):i53-54. Available at: https://academic.oup.com/humrep/article/32/suppl_1/i1/3926642 [Last accessed: May 2022].
- Duijkers IJM, Klingmann I, Prinz R, Hrafnsdottir S, Magnusdottir ThB, Klipping C. Effect on endometrial histology and pharmacokinetics of different dose regimens of progesterone vaginal pessaries, in comparison with progesterone vaginal gel and placebo. Human Reproduction 2018;33:2131-2140. Available at: https://academic.oup.com/humrep/article/33/11/2131/5108541 [Last accessed: April 2022].
- Aghsa MM, Rahmanpour H, Bagheri M, Davari-Tanha F, Nasr R. A randomized comparison of the efficacy, side effects and patient convenience between vaginal and rectal administration of Cyclogest® when used for luteal phase support in ICSI treatment. Archives of Gynecology and Obstetrics 2012;286:1049–1054. Available at: https://link.springer.com/article/10.1007/s00404-012-2410-7 [Last accessed: April 2022].
- Khrouf M, Slimani S, Khrouf MR, Braham M, Bouyahia M, Berjeb KK, Chaabane HE, Merdassi G, Kaffel AZ, Zhioua A, Zhioua F. Progesterone for Luteal Phase Support in In Vitro Fertilization: Comparison of Vaginal and Rectal Pessaries to Vaginal Capsules: A Randomized Controlled Study. Clinical Medicine Insights: Women’s Health 2017;9:43-47. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5217976/ [Last accessed: April 2022].
- McAuley JW, Kroboth FJ, Kroboth PD. Oral Administration of Micronized Progesterone: A Review and More Experience. Pharmacotherapy 1996;16:453-457. Available at: https://accpjournals.onlinelibrary.wiley.com/doi/abs/10.1002/j.1875-9114.1996.tb02977.x?sid=nlm%3Apubmed [Last accessed: April 2022].
- Afridi N, Masood U, Balooch S, Khan S, Khan S. Comparison of efficacy of oral progesterone and micronized progesterone pessary in reduction of incidence of spontaneous preterm births. Journal of Ayub Medical College Abbottabad 2019;31:248-251. Available at: https://jamc.ayubmed.edu.pk/jamc/index.php/jamc/article/view/6187/2681 [Last accessed: April 2022].
- Saunders H, Khan C, D’Hooghe T, Magnúsdóttir TB, Klingmann I, Hrafnsdóttir S. Efficacy, safety and tolerability of progesterone vaginal pessaries versus progesterone vaginal gel for luteal phase support after in vitro fertilisation: a randomised controlled trial. Human Reproduction 2020;35:355-363. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7048710/ [Last accessed: April 2022].
- Mesen TB, Young SL. Progesterone and the Luteal Phase: A Requisite to Reproduction. Obstetrics and Gynecology Clinics of North America 2015;42:135-151. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0889854514000965?via%3Dihub [Last accessed: April 2022].
- van der Linden M, Buckingham K, Farquhar C, Kremer JAM, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database of Systematic Reviews 2015. Available at: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009154.pub3/full [Last accessed: April 2022].
- Unfer V, di Renzo GC, Gerli S, Casini ML. The Use of Progesterone in Clinical Practice: Evaluation of its Efficacy in Diverse Indications Using Different Routes of Administration. Current Drug Therapy 2006;1:211-219. Available at: https://www.eurekaselect.com/public/article/797 [Last accessed: April 2022].
- Cable JK, Grider MH. Physiology, Progesterone. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK558960/ [Last accessed: March 2022].
- Wetendorf M, DeMayo FJ. Progesterone receptor signaling in the initiation of pregnancy and preservation of a healthy uterus. The International Journal of Developmental Biology 2014;58:95-106. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4413906/ [Last accessed: March 2022].
- Malik S, Krishnaprasad K. Natural Micronized Progesterone Sustained Release (SR) and Luteal Phase: Role Redefined!! Journal of Clinical & Diagnostic Research 2016;10:QE01-QE04. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4800604/ (Last accessed: March 2022].
- Cédrin-Durnerin I, Isnard T, Mahdjoub S, Sonigo C, Seroka A, Comtet M, Herbemont C, Sifer C, Grynberg M. Serum progesterone concentration and live birth rate in frozen-thawed embryo transfers with hormonally prepared endometrium. Reproductive Biomedicine Online 2019;38(3):472-480. Available at: https://pubmed.ncbi.nlm.nih.gov/30642638/ [Last accessed: April 2022].
- Labarta E, Mariani G, Paolelli S, Rodriguez-Varela C, Vidal C, Giles J, Bellver J, Cruz F, Marzal A, Celada P, Olmo I, Alamá P, Remohi J, Bosch E. Impact of low serum progesterone levels on the day of embryo transfer on pregnancy outcome: a prospective cohort study in artificial cycles with vaginal progesterone. Human Reproduction 2020;36:683-692. Available at: https://pubmed.ncbi.nlm.nih.gov/33340402/ [Last accessed: April 2022].
- Devall AJ, Papadopoulou A, Podesek M, Haas DM, Price MJ, Coomarasamy A, Gallos ID. Progestogens for preventing miscarriage: a network meta-analysis. Cochrane Databse of Systematic Reviews 2021. Available at: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013792.pub2/full [Last accessed: April 2022].
- National Institute for Health and Care Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management, 2019. Available at: https://www.nice.org.uk/guidance/ng126 [Last accessed: April 2022].
- National Institute for Health and Care Excellence. Preterm labour and birth, 2015. Available at: https://www.nice.org.uk/guidance/ng25/chapter/Recommendations [Last accessed: April 2022].
- Cyclogest 400mg pessaries. Patient Information Leaflet. Available at: https://www.medicines.org.uk/emc/product/5569/pil [Last accessed: April 2022].
- Ghanem ME, Al-Boghdady LA. Luteal Phase Support in ART: An Update. In: Enhancing success of Assisted Reproduction [Internet]. IntechOpen, 2012. Available at: https://www.intechopen.com/chapters/41085 [Last accessed: April 2022].
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5994, Progesterone, 2022. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Progesterone [Last accessed: April 2022].
- Yovich JL, Conceicao JL, Stanger JD, Hinchliffe PM, Keane KN. Mid-luteal serum progesterone concentrations govern implantation rates for cryopreserved embryo transfers conducted under hormone replacement. Reproductive BioMedicine Online 2015;31: 180-191. Available at: https://www.rbmojournal.com/article/S1472-6483(15)00243-6/fulltext [Last accessed: April 2022].
- Labarta E, Mariani G, Holtmann N, Celada P, Remohi J, Bosch E. Low serum progesterone on the day of embryo transfer is associated with a diminished ongoing pregnancy rate in oocyte donation cycles after artificial endometrial preparation: a prospective study. Human Reproduction 2017;32:2437-2442. Available at: https://academic.oup.com/humrep/article/32/12/2437/4508784 [Last accessed: April 2022].

