I'm a fertility HCP
An overview of fertility
Infertility is a multifactoral disease with more and more treatments taking place.1,2 Common causes of female infertility include ovulatory dysfunction, and tubal, uterine and peritoneal factors.1 In the male, common causes include chromosomal abnormalities and testicular injury.3 However, many cases remain unexplained.1,3
It is estimated that globally around 48 million couples and 186 million individuals are affected by infertility.4 Fertility treatment rates will continue to grow as an increasing number of women utilise assisted reproductive technology, such as in vitro fertilisation, to achieve a successful pregnancy.2
In most stimulated assisted reproductive technology cycles, the luteal phase of the menstrual cycle is compromised due to the administration of gonadotropins (primarily follicle stimulating hormone to increase the number of oocytes collected), reducing the likelihood of a successful pregnancy.5
When stimulating the ovaries to create multifollicular development, the fast-rising oestradiol levels may elicit an untimely luteinising hormone surge at a time when follicles may not have grown sufficiently large to release the best quality oocytes, resulting in a failed oocyte pick-up procedure. The use of agents to prevent this luteinising hormone surge (such as gonadotropin-releasing hormone agonists, gonadotropin releasing hormone antagonists and progestins) has almost completely prevented this from occurring.6
The reduced levels of luteinising hormone inhibit the release of progesterone from the corpora lutea, which is required for secretory changes in the mucous membrane of the uterus and successful implantation of the embryo.7 This consequently reduces the likelihood of a successful pregnancy.5 Therefore, luteal phase support is an essential component of assisted reproductive technology for all patients.8
Understanding the luteal phase
The luteal phase occurs in the second half of the menstrual cycle, following ovulation.9 Here, the residual follicle transforms into the corpus luteum, which secretes progesterone and some oestrogen preventing further ovulation within the cycle.9 Progesterone promotes changes in the endometrium that facilitate implantation of the blastocyst and provide nutrients for the growing embryo.9
In many patients undergoing assisted reproductive technology, the luteal phase is disrupted leading to low progesterone levels10 and reduced pregnancy rates.5 Luteal Phase Support is therefore required to overcome this.

Luteal Phase Support in Assisted Reproductive Technology with progesterone
During luteal phase defect may occur following controlled ovarian hyperstimulation and oocyte aspiration, which can suppress pituitary secretion of luteinising hormone. The suppression of luteinising hormone in turn affects the release of progesterone by the corpus luteum, which is essential for embryo implantation and development in the uterine lining10; this reduces the likelihood of a successful pregnancy.5
Due to the reduced levels of progesterone in women undergoing assisted reproductive technology, support during the luteal phase is required to overcome this. Luteal Phase Support involves the administration of hormones, such as progesterone, to improve implantation and pregnancy rates.11
Progesterone is recommended by the National Institute for Health and Care Excellence (NICE) for Luteal Phase Support in assisted reproductive technology12 and contributes to an increase in successful pregnancy and live birth rates.11 It overcomes the insufficient levels of progesterone in assisted reproductive technology patients by stimulating progesterone secretion and supporting embryo implantation.11
Progesterone is a naturally occurring hormone during pregnancy and poses no known additional risks when administered to women during the first trimester following assisted reproductive technology.13

Adapted from the Atlas of Human Assisted Reproductive Technologies.14
Route of administration for Luteal Phase Support
Luteal phase support with progesterone can be administered by the following routes:
Vaginal
- Effective8
- Provides ideal progesterone concentrations14
- Convenient14
- Most used progesterone regimen in Europe11
- Demonstrated safety profile15
Rectal
- Effective14
- May be used as an alternative to the vaginal route, where the patient has experienced previous perineal irritation14
Intramuscular8
- Effective17
- Painful
- Cannot be self-administered
- Possibility of serious local reactions
Oral
- Patient-friendly16
- Low bioavailability (10%)8 due to intense liver metabolism17
- Provides ineffective Luteal Phase Support17
- Increased incidence of somnolence17 and systemic side effects18
Vaginally administered progesterone: the preferred choice for Luteal Phase Support
Vaginal administration can be an effective8 and convenient14,19 choice for progesterone delivery in Luteal Phase Support for assisted reproductive technology patients.8,14 A recent survey published in 2020 indicated that 80% of clinicians worldwide choose to solely use vaginal progesterone,16 whilst another demonstrated that the vaginal route has remained clinicians choice of Luteal Phase Support regimen from 2009 to 2019.17
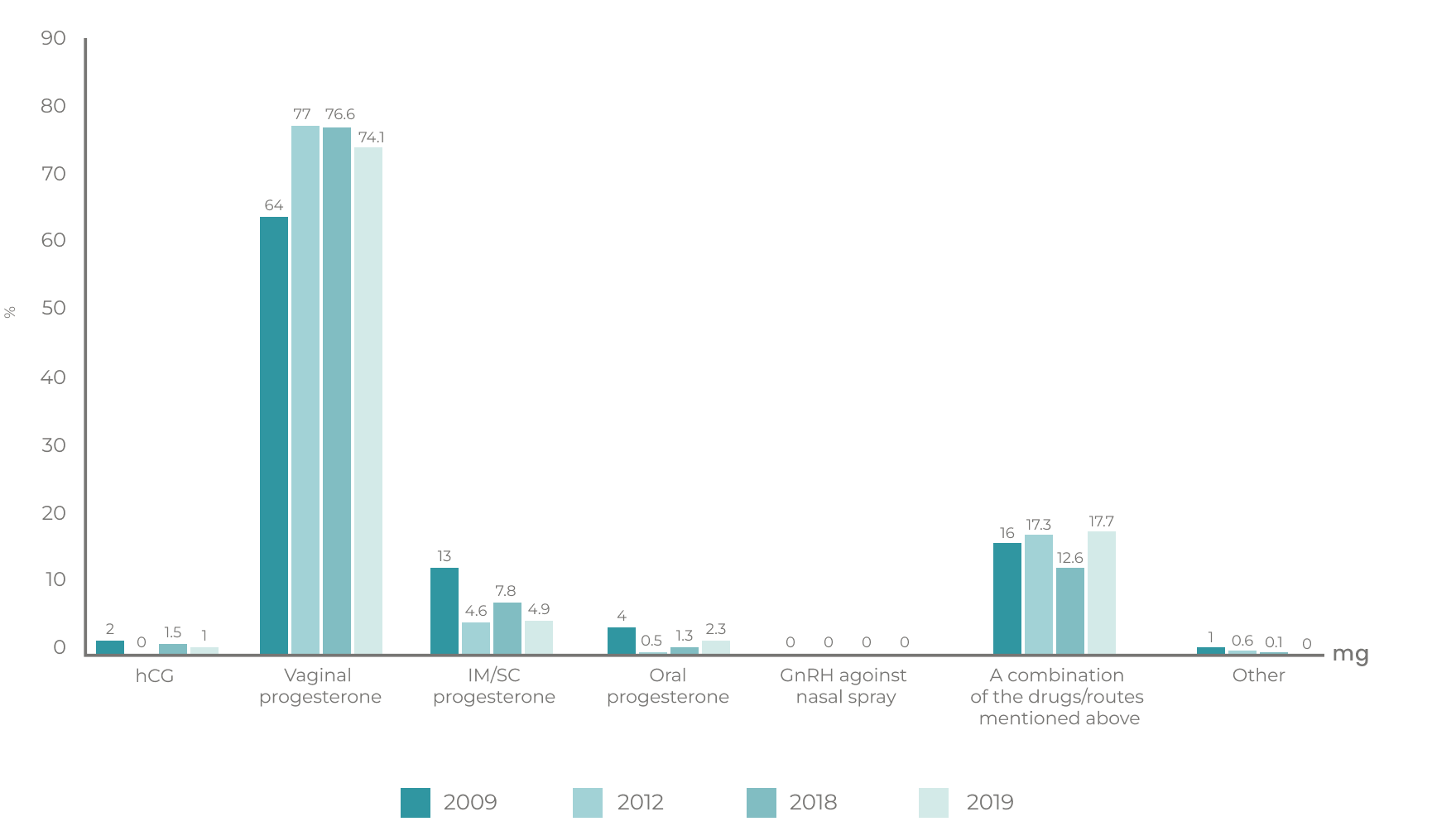
Adapted from: Shoham, et al., Reprod Biol Endocrinol 2021.17
Benefits of vaginal progesterone:
- Higher endometrial tissue levels of progesterone.20
- Results in adequate secretory endometrial transformation.20
- Vaginal delivery avoids first-pass metabolism, which improves the bioavailability of vaginal compared to oral formulations.20
- Long-term experience demonstrates a well-known safety profile.15,21
- Minimises systemic side effects.20
- Avoids pain and anxiety of injection.19
- Cyclogest® vaginal pessaries are a well-tolerated and effective medication for Luteal Phase Support in ART.22
- Cyclogest® is suitable for vegan and vegetarians. This is certified with the Vegan Society.

- Aali BS, Ebrahimipour S, Medhdizadeh S. The effectiveness of luteal phase support with cyclogest in ovarian stimulated intra uterine insemination cycles: A randomized controlled trial. Iranian Journal of Reproductive Medicine 2013;11:309-314. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3941431/ [Last accessed: December 2023]
- Ferraretti AP, Nygren A, Andersen N, de Mouzon J, Kupka M, Calhaz-Jorge C, Wyns C, Gianaroli L, Goossens V. Trends over 15 years in ART in Europe: an analysis of 6 million cycles. Human Reproduction Open 2017;2017 Issue 2, 2017,1-10. Available at: https://academic.oup.com/hropen/article/2017/2/hox012/4096838 [Last accessed: December 2023].
- Punab M, Poolamets O, Paju P, Vihljajev V, Pomm K, Ladva R, Korrovits P, Laan M. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Human Reproduction 2017;32:18-31. Available at: https://academic.oup.com/humrep/article/32/1/18/2605950#57954935 [Accessed at: December 2023].
- World Health Organization. Infertility. Available at: https://www.who.int/news-room/fact-sheets/detail/infertility [Last accessed: December 2023].
- Mesen TB, Young SL. Progesterone and the Luteal Phase: A Requisite to Reproduction. Obstetrics and Gynecology Clinics of North America 2015;42:135-151. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0889854514000965?via%3Dihub [Last accessed: December 2023].
- European Society of Human Reproduction and Embryology. Guidelines on Ovarian Stimulation for IVF/ICSI. Available at: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Ovarian-Stimulation-in-IVF-ICSI [Last accessed: December 2023].
- Czyzyk A, Podfigurna A, Genazzani AR, Meczekalski B.The role of progesterone therapy in early pregnancy: from physiological role to therapeutic utility. Gynecological Endocrinology 2017;33:421-424. Available at: https://www.tandfonline.com/doi/full/10.1080/09513590.2017.1291615 [Last accessed: December 2023].
- Vaisbuch E, de Ziegler D, Leong M, Weissman A, Shoham Z. Luteal-phase support in assisted reproduction treatment: real-life practices reported worldwide by an updated website-based survey. Reproductive Biomedicine Online 2014;28:330-5. Available at: https://pubmed.ncbi.nlm.nih.gov/24447959/ [Last accessed: December 2023].
- Reed BG, Carr BR. The Normal Menstrual Cycle and the Control of Ovulation. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available at: https://www.ncbi.nlm.nih.gov/books/NBK279054/ [Last accessed: December 2023].
- Tesarik J, Conde-Lopez C, Galan-Lazaro M, Mendoza-Tesarik R. Luteal Phase in Assisted Reproductive Technology. Frontiers Reproductive Health 2020;2. Available at: https://www.frontiersin.org/articles/10.3389/frph.2020.595183/full [Last accessed: December 2023].
- van der Linden M, Buckingham K, Farquhar C, Kremer JAM, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database of Systematic Reviews 2015. Available at: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009154.pub3/full [Last accessed: December 2023].
- National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. Available at: https://www.nice.org.uk/guidance/cg156/chapter/recommendations [Last accessed: December 2023].
- Child T, Leonard SA, Evans JS, Lass A. Systematic review of the clinical efficacy of vaginal progesterone for luteal phase support in assisted reproductive technology cycles. Reproductive Biomedicine Online 2018;36:630-645. Available at: https://www.rbmojournal.com/article/S1472-6483(18)30055-5/fulltext [Last accessed: December 2023].
- Khrouf M, Slimani S, Khrouf MR, Braham M, Bouyahia M, Berjeb KK, Chaabane HE, Merdassi G, Kaffel AZ, Zhioua A, Zhioua F. Progesterone for Luteal Phase Support in In Vitro Fertilization: Comparison of Vaginal and Rectal Pessaries to Vaginal Capsules: A Randomized Controlled Study. Clinical Medical Insights: Women’s Health 2016;9:43-47. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5217976/ [Last accessed: December 2023].
- Duijkers IJM, Klingmann I, Prinz R, Wargenau M, Hrafnsdottir S, Magnusdottir TB, Klipping C. Effect on endometrial histology and pharmacokinetics of different dose regimens of progesterone vaginal pessaries, in comparison with progesterone vaginal gel and placebo. Human Reproduction 2018;33:2131-2140. Available at: https://academic.oup.com/humrep/article/33/11/2131/5108541?login=false [Last accessed: December 2023].
- Di Guardo F, Midassi H, Racca A, Tournaye H, De Vos M, Blockeel C. Luteal Phase Support in IVF: Comparison Between Evidence-Based Medicine and Real-Life Practices. Frontiers in Endocrinology 2020;11. Available at: https://www.frontiersin.org/articles/10.3389/fendo.2020.00500/full
- Shoham G, Leong M, Weissman A. A 10-year follow-up on the practice of luteal phase support using worldwide web-based surveys. Reproductive Biology and Endocrinology 2021;19:15. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7836509/ [Last accessed: December 2023].
- Ludwig M, Diedrich K. Evaluation of an optimal luteal phase support protocol in IVF. Acta Obstetricia et Gynecologica Scandinavica 2001;80:452-466. Available at: https://obgyn.onlinelibrary.wiley.com/doi/full/10.1034/j.1600-0412.2001.080005452.x [Last accessed: December 2023].
- Zaman AY, Coskun S, Alsanie AA, Awartani KA. Intramuscular progesterone (Gestone) versus vaginal progesterone suppository (Cyclogest) for luteal phase support in cycles of in vitro fertilization–embryo transfer: patient preference and drug efficacy. Fertility Research and Practice 2017;3:17. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5679140/ [Last accessed: December 2023].
- Bulletti C, de Ziegler D, Flamigni C, Giacomucci E, Polli V, Bolelli G, Franceschetti F. Targeted drug delivery in gynaecology: the first uterine pass effect. Human Reproduction 1997;12:1073-1079. Available at: https://academic.oup.com/humrep/article/12/5/1073/665486?login=false [Last accessed: December 2023].
- Piette PCM. The Pharmacodynamics and Safety of Progesterone. Best Practice and Research in Clinical Obstetrics and Gynaecology 2020;69:13-29. Available at: https://pubmed.ncbi.nlm.nih.gov/32739288/ [Last accessed: December 2023].
- Saunders H, Khan C, D’Hooghe T, Magnúsdóttir TB, Klingmann I, Hrafnsdóttir S. Efficacy, safety and tolerability of progesterone vaginal pessaries versus progesterone vaginal gel for luteal phase support after in vitro fertilisation: a randomised controlled trial. Human Reproduction 2020;35:355-363. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7048710/ [Last accessed: December 2023].
- Atlas of Human Assisted Reproductive Technologies Jaypee Brothers Medical Publishers (P) Ltd.; 1st edition (30 Nov. 2006).
- Zaman et al. Fertility Research and Practice (2017) 3:17 Intramuscular progesterone (Gestone) versus vaginal progesterone suppository (Cyclogest) for luteal phase support in cycles of in vitro fertilization–embryo transfer: patient preference and drug efficacy DOI 10.1186/s40738-017-0044-y

